Children’s Inn Residents Participate in NCI’s Center for Cancer Research Trial That Shrinks Tumors in Children with NF1
Findings from a phase 2 clinical trial show that the drug selumetinib improves outcomes for children with the genetic disorder neurofibromatosis type 1 (NF1).
In the trial, selumetinib shrank the inoperable tumors that develop with NF1 called plexiform neurofibromas, and children experienced reduced pain, improved function, and better overall quality of life after receiving the treatment.
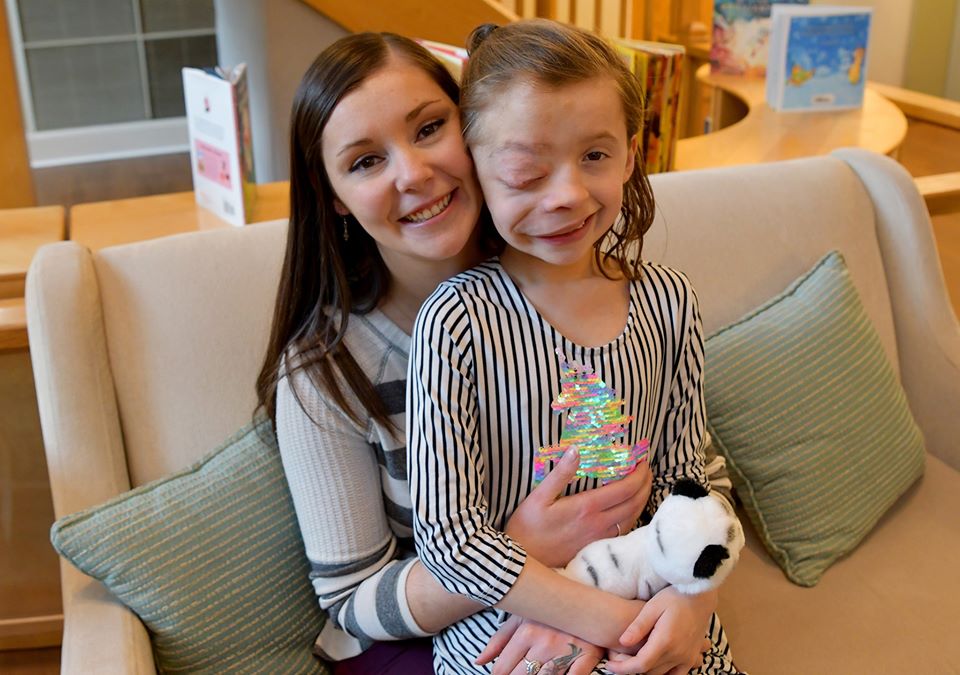
Autumn, 9, who has neurofibromatosis type 1, and her mom, Lindsay, of Kansas, have been coming to The Children’s Inn since Autumn was 2 to participate in NCI clinical research studies. The NCI trial medication is keeping Autumn’s tumor stable.
The study was conducted at several sites throughout the country, including the NIH Clinical Center in Bethesda, Maryland, with the help of a large number of children who regularly stay at The Children’s Inn at NIH to make their trial participation possible and comfortable.
The study was published March 18, 2020, in the New England Journal of Medicine. Read NCI’s full press statement about its successful trial.
NIH Director Dr. Francis Collins recently penned an article on his blog highlighting this medical breakthrough. Read here: “Encouraging News for Kids with Neurofibromatosis Type 1.”

Xavier, 12, of Pennsylvania has been participating in NCI’s selumetinib trial for more than one year and stayed at The Children’s Inn several times with his mom, Seddra. Xavier has experienced significant shrinkage of the tumors caused by NF1 that were encroaching upon his spine.
The Children’s Inn thanks you for your donations that make it possible for children and young people to stay free of charge at The Inn so they can participate in groundbreaking NIH clinical trials that help bring about new treatments and cures to benefit children today and generations to come.




