FDA Approves First Drug to Treat Inoperable Tumors in Children and Young Adults With NF1
Children and Young Adults staying at The Children’s Inn Played Instrumental Role in Trial
The Food and Drug Administration (FDA) has approved the first medication to treat inoperable tumors in children and young adults resulting from neurofibromatosis type 1, a genetic tumor predisposition disorder. The medication has been extensively tested by researchers at the National Cancer Institute with the help of children and young adults with NF1 staying at The Children’s Inn at NIH as well as patients in other locations.
“Until now, no effective medical therapies have existed for children with NF1 and plexiform neurofibromas, and it’s been a long journey to find a drug that can help them,” says Brigitte Widemann, M.D., principal investigator of the study, and chief of the National Cancer Institute’s Pediatric Oncology Branch at the Center for Cancer Research, which developed and coordinated the trial. “While this is not yet a cure, this treatment is shrinking tumors and it’s making children feel better and have a better quality of life.”
Children with NF1 have been coming to The Children’s Inn from as far as Utah, Kansas and Alaska to participate in the promising clinical research study. Their symptoms included pain, facial or other disfigurement, neurological problems or the threat of neurological problems due to growing tumors. Trial data show approximately 70% of participants achieved tumor shrinkage greater than 20%, and that most maintained the shrinkage for more than one year.
“The trials that we have designed are for tumors called plexiform neurofibromas,” Andrea Gross, M.D., lead author of the study and an assistant research physician at NCI’s Center for Cancer Research’s Pediatric Oncology Branch, says. “They are not cancer tumors, but they can cause lots and lots of medical problems like pain, disfigurement, problems breathing and decrease in strength or range of motion.”
The FDA’s approval of the trial drug brings hope to children and young adults with NF1 who experience severe symptoms – children like Inn resident Autumn, 9, of Kansas, who has participated in NCI’s NF1 trials since she was 2 years old. Autumn and her family have stayed at The Children’s Inn numerous times to make Autumn’s trial participation possible.
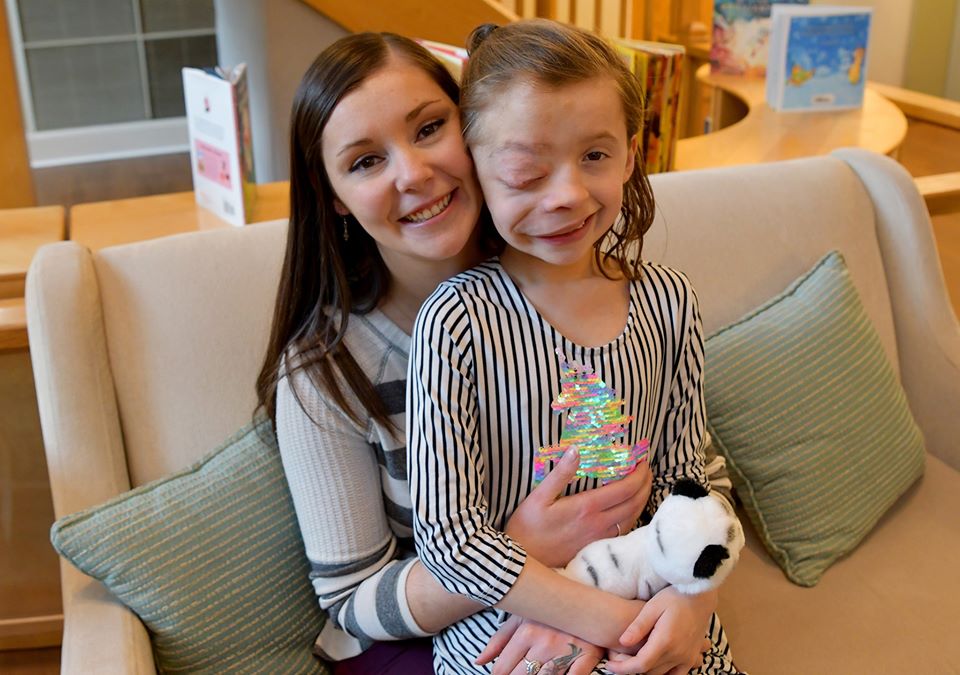
Autumn with her mom, Lindsay.
“Dr. Widemann has been her doctor since she was 2 years old,” Autumn’s mom, Lindsay, says. “We really feel like we have an advocate here that’s fighting for not just NF1 but Autumn specifically.
“When she’s not on the medicine, her tumor grows dramatically,” her mom adds. “So when she is on the medicine, even if it’s not shrinking, at least it’s stable.”
For Children’s Inn resident Xavier, 12, of Pittsburgh, Pennsylvania, who has been participating in NCI’s selumetinib trial for NF1 for more than one year, shrinkage of the tumors around his spine helps protect him from possible neurological problems including paralysis. His mom calls the NCI trial “a lifesaver.” Read Xavier’s full story here.

Xavier and his mom Seddra.
“We are honored to provide free lodging and support to children like Autumn, Xavier and others who are helping make medical discoveries possible,” says Children’s Inn CEO Jennie Lucca. “The Children’s Inn’s goals are to make childhood possible today and a treatment or cure possible tomorrow. The FDA’s approval of this breakthrough medication is great news for the children on this important trial and everyone affected by NF1.”
Please read an NCI blog post here about the clinical research study that led to the landmark FDA approval.
Your support makes it possible for children like Autumn and Xavier to stay at The Children’s Inn while they seek life-changing treatment at the NIH.